Turning Early Funding Into Evidence and Impact
Framework for Moving from Capital to Market
Congratulations! Securing early funding is a huge milestone. You’ve secured the fuel to move your idea forward, and the future looks exciting. But if you’re like many founders, the next thought is: now what? The product vision is crystal clear in your head. But here’s the reality check- without a clear regulatory and evidence strategy, even the most brilliant innovation can stall before it reaches the market.
This is where things can get tricky. Moving from funding to a product on the market isn’t straightforward. It’s full of critical decisions: Where do you focus first? How do you avoid burning through cash too quickly? What evidence will regulators, payers, and investors expect?
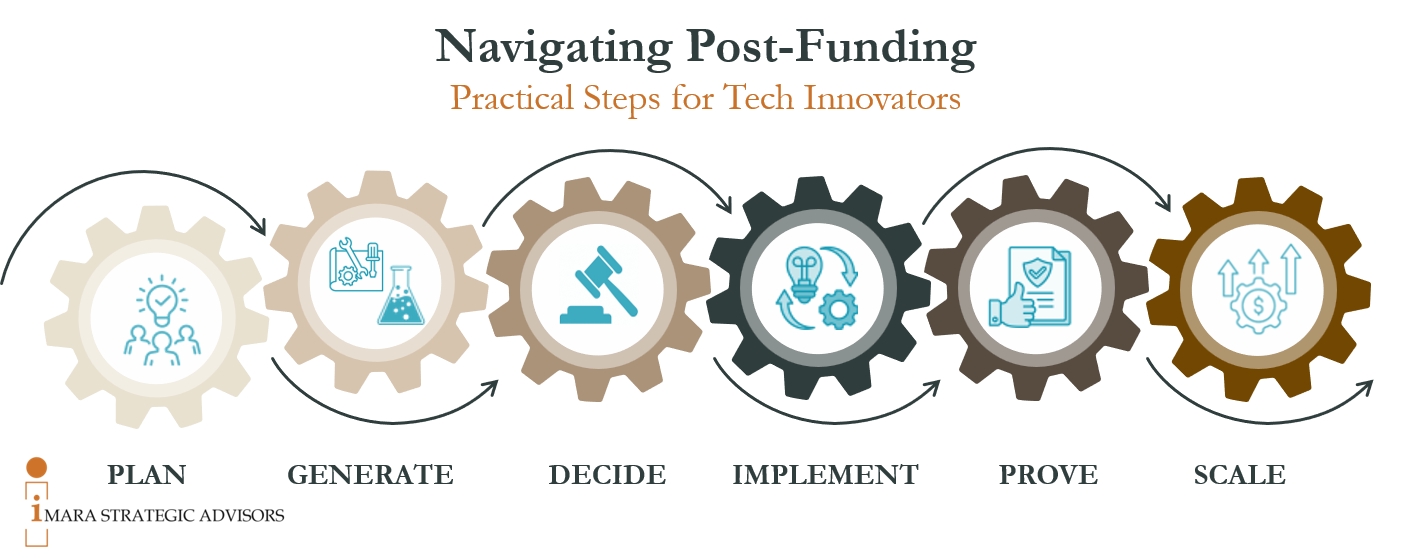
That’s where thinking in terms of an Evidence Lifecycle can help. At Imara, we use our own Evidence Lifecycle the PGDIPS framework; six interconnected steps that guide your strategy; Plan, Generate, Decide, Implement, Prove, and Scale.
Step 1: Plan- Build Your Strategic Roadmap
This is where you set your foundation for success. Think of this as laying the blueprint for your innovation. Before your first prototype or pilot study, you need clarity on:
Which regulatory pathways are realistic
What evidence regulators and stakeholders will expect
How your clinical, technical, and commercial strategies intersect.
Planning early avoids costly pivots later and gives your team strategic direction from day one
Step 2: Generate - Gather the Right Evidence at the Right Time
Success depends on gathering the right data not just more data. Once you’ve mapped out the strategy, the next step is evidence generation. Too much early-stage data can slow you down just as easily as too little. The key isn’t to collect everything; it’s to collect what matters most.
What’s the minimum evidence you need to move forward confidently?
Which questions will investors, regulators, and customers ask first?
How can you design data plans that support multiple future uses?
With a clear evidence plan, you avoid the trap of “research for research’s sake” and generate insights that actually move the needle. Focus on collecting fit-for-purpose evidence that helps you unlock your next milestone.
Step 3: Decide - Use Evidence to Drive Confident Choices
At some point, you’ll face make-or-break decisions:
Do we invest in another round of testing?
Is our design ready for clinical studies?
Do we pursue this indication or pivot?
The Decide phase is where evidence earns its value. An evidence plan that’s done right enables you to make smarter, risk-balanced choices. Having your regulatory and evidence priorities mapped out makes these decisions faster, clearer, and less risky. Your roadmap acts as a built-in decision-support tool; keeping leadership aligned and focused.
Step 4: Implement - Turn Strategy Into Action
Once the plan is set, it’s time to operationalize. That means getting your cross-functional teams- R&D, regulatory, quality, and clinical- pulling in the same direction.
With aligned milestones, you move forward confidently, avoid duplicate efforts, and reduce the risk of last-minute surprises when approaching submissions or investor pitches. The Implement phase is about translating plans into coordinated action - launching studies, preparing submissions, and aligning your teams.
Step 5: Prove - Demonstrate Value and Build Trust
Now is the time to tell a compelling evidence story. Evidence is only as powerful as the story it tells. When it’s time to engage regulators, investors, or partners, evidence is your currency. But data alone isn’t enough; it needs to be structured into a cohesive, credible story:
Why your product is safe
How it delivers value
Where it fits into the market
By thinking about this from day one, you ensure your evidence package is built to persuade; not patched together at the finish line.
Step 6: Scale - Leverage Evidence for Growth
Getting to market is just the beginning. The Evidence Lifecycle doesn’t stop at approval. A smart roadmap allows you to design for long term impact and sets you up to:
Plan post-market evidence generation
Expand claims and indications
Support global regulatory submissions
Startups that think beyond the first launch avoid getting boxed in by short-term choices and position themselves for sustainable growth.
The Takeaway
Early funding gives you the momentum; but how you use it determines your success. The Imara Evidence Lifecycle (PGDIPS) provides structure for moving forward strategically, with clarity and confidence.
The most successful startups don’t just build products — they build strategies. A clear regulatory and evidence roadmap gives you more than compliance; it gives you clarity, speed, and confidence.
With the right planning, you can reduce risk, accelerate decision-making, and tell a stronger story to every stakeholder who matters.